Healthcare Textiles: "Guardians of Clean: The Vital Role of Process Monitoring in Healthcare Linen Hygiene"
As we have emphasized in earlier posts, cleaning healthcare textiles and protecting them up to the point of patient use is a complicated process with many steps, spanning the hospital environment, the laundry, and even transportation services. Each step must be rigorously executed. One mis-executed step can result in linen contamination leading to patient infection or even death! In this final post, we discuss how to verify that all steps are executed as specified ensuring a safe outcome every time? We will discuss ways of building processes around monitoring our processes.
We are all inundated daily with studies, claims, certifications, and registrations about infection prevention in the healthcare setting. While it is generally understood that proper healthcare textile hygiene must be an integral part of any infection prevention strategy, here are some common assertions and their claims:
Claim 1: Our surface disinfectant kills 99.99% of germs!
Claim 2: Our laundry chemical is certified to kill SARS-CoV-2, the virus that causes COVID-19.
Claim 3: Our laundry plant is certified. We tested samples of finished linen and their CFU counts are < 20.
Claim 4: The air that touches our clean healthcare textiles is clean enough!
Claim 5: Our finished linen treatment chemical kills C-Diff!
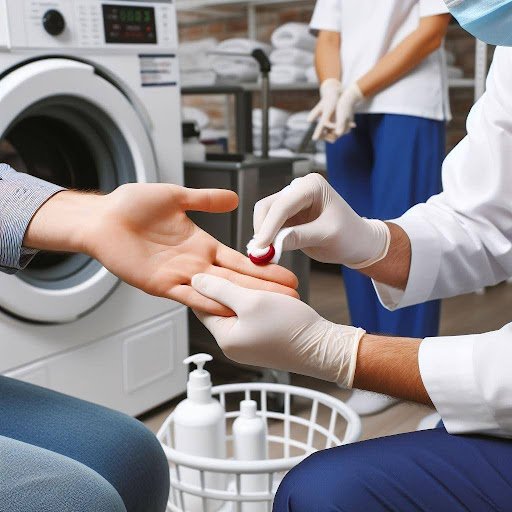
Figure 1: Measuring Linen for Bioburden
We are living in skeptical times, which forces us to ask: Are these baseless claims or should we trust them? So many questions come to mind.
For example, regarding the first claim: Which germs does the disinfectant kill or arguably more important, which does it not kill? What about the other .01% of germs? How must this disinfectant be used?
Regarding the second claim: Does that mean if we do not use your laundry chemical, we can’t kill SARS-CoV-2? Will standard healthcare laundry processes kill SARS-CoV-2? Does the third claim mean that all my healthcare textiles (HCTs) are clean enough? What do the popular third-party certifications and their designations mean and how are they measured? In the fourth claim, what does “clean enough” mean for air? How was it measured? Is all the air touching clean HCT clean enough? And lastly, the fifth claim, does having to use a finished linen treatment chemical to kill C-Diff spores mean that standard healthcare laundry processes do not work?
The following seeks to answer questions like the above and to inform on what can be done to verify the accuracy of our healthcare linen processes. In doing so, we hope to convince you of the importance of monitoring laundry processes to consistently provide hygienically clean and safe HCTs for sick patients. While there are established standards and best management practices for HCT processing (see: http://HLACNET.Org or Healthcare – Hygienically Clean ) we need to go further and monitor the process to give confidence in the finished product.
We know that microorganisms are ubiquitous. There is essentially no environment where microorganisms cannot exist. Therefore, our goal must be to maintain an environment of high microbial integrity around our HCTs.
The Problem – No Agreed Upon Measures of Contamination
The problem is there is generally a lack of agreed upon standards of cleanliness in healthcare linen processes. There is no definition of hygienically clean linen, carts, water, air, surfaces, and even employee hands. Furthermore, there are no standard methods of measurement of linen processes. This leads to such logical questions as:
How clean is clean enough? (This was discussed in the February 2020 Issue of Home - Healthcare Hygiene magazine.)
What contamination values are acceptable throughout the process? There are lots of variables.
Do HCTs need to be sterile in certain areas?
Is my laundry processing equipment performing?
Are my laundry people performing?
Are my laundry processes performing?
Clearly, without a comprehensive process monitoring program around our HCT system and without objective measures of contamination, we are, at best, operating in a snapshot in time. At worst we are effectively operating blindly.
The Solution – Process Monitoring
Therefore, the first step is to define the areas to test, the testing frequency, the testing methodology, and the acceptable contamination values for each. Important categories to test in any environment where HCT are found include the surfaces that touch clean linen; air; laundry process water; Linen staff hands; and the finished HCT product.
Specifically:
Surfaces that touch clean linen
Washed HCTs can become re-contaminated when in contact with dirty surfaces such as those found in a laundry or a hospital. These include folding tables, linen conveyors, linen shelves, and clean linen carts. We cannot realistically test every surface every minute of every day. Therefore, it is a good idea to choose a variety of key surfaces and regularly test for contamination. Define the test method and define the acceptable contamination values. More on this later. Contamination values found above acceptable levels mean cleaning protocols need to be re-evaluated. Disinfectants or cleaning equipment may need to be adjusted (equipment); Cleaning frequency may need to be increased (process); or cleaning personnel may need additional training (people).
Figure 2: Measuring a Healthcare Surface for Contamination
Air
Clean HCTs are exposed to air the instant they leave the washer and remain exposed for the duration of their journey to the patient. Contaminated air can contaminate HCT. Lint and dust can be especially problematic. Areas of a laundry especially susceptible to dirty air include dryers, ironer and piece folder areas, and hand folding stations. In a hospital, check linen rooms and linen closets. As with surfaces, choose a variety of locations and regularly test for contamination. Define the test method and define the acceptable contamination values. Contamination values found above acceptable levels mean air cleaning protocols need to be re-evaluated. Air filters or air handlers may need adjustment (equipment); Blowdown frequency may need to be increased (process); or cleaning personnel may need additional training (people)
Figure 3: Measuring the air for Contamination in a Healthcare Laundry
Process water
Clean process water is a necessary ingredient in producing clean HCTs. Good places to test process water cleanliness include the main city water supply, cold process water tanks, and final rinse water zones in a tunnel washer. Water should be tested at least monthly and acceptable contamination values should be set. Water contamination values found above acceptable levels mean water protocols need to be re-evaluated. Dirty city water may need additional filtration or treatment (equipment); Dirty clean water tank storage means tank cleaning procedures must be re-evaluated (people/process); Dirty final rinse zones means dirty water will re-contaminate HCT. Tunnel tank cleaning protocols must be re-evaluated and personnel re-trained on these (people/process/equipment)
Employee hands
Hospital personnel and laundry personnel often touch clean HCT. If their hands are dirty, they will re-contaminate the clean HCT. While it is unrealistic to test every employee’s hands every minute of every day, it is a good idea to sample a few employee hands regularly. Define the test method and define the acceptable contamination values. Contaminated hand values found above acceptable levels mean hand hygiene protocols, equipment, and training must be reassessed (people/process/equipment).
Figure 4: Are this Worker's Hands Clean?
Finished HCT
The final step in a linen process monitoring program is to test the bioburden levels on the finished HCT. Once again, strategic sampling is our best solution. Choose several different textile blends including 100% cotton items (e.g., OR Towels), polyester cotton blends (e.g. blankets, sheets, towels), and 100% synthetic items (e.g., surgery gowns, isolation gowns). Test regularly and be sure they are sampled as close to patient end use as possible. Define the test method and define the acceptable contamination values. Contaminated HCT values found above acceptable levels mean the process needs to be re-assessed. First test the HCT out of the wash to ensure they are within acceptable levels. If not, there is an issue with the wash process that must be addressed. If HCT out of the wash are clean, then the problem lies downstream. Check air, surface, and employee hands for areas where re-contamination may be occurring. Hopefully, our other process monitoring steps have already identified these.
How to test? Tap the latest resources
Any tests for contamination are only as good as the testing methodology employed. Some key test areas to look at include test accuracy/detail, test repeatability, organisms identified, timeliness of results, test labor intensity, and test cost. A test that can identify actual microorganisms is a benefit and can answer some of our questions above. That way, it is possible to determine if your process is killing targeted microorganisms such as SARS-CoV-2 or C-Diff. Tests that can be performed by your staff onsite will be more timely and less costly. Tests that are more thorough and identify more CFU will be more accurate. A superficial bioburden test that shows low CFU but misses many microorganism colony counts gives a false sense of security. Be sure to understand the above when choosing your testing methodology.
How do we define acceptable contamination levels?
Generally, acceptable contamination criteria do not exist yet for healthcare linens. Consequently, they must be defined internally and then measured against. As more hospitals and laundries implement process monitoring and more data is gathered, we can develop more accurate contamination standards. It is a good idea to involve hospital infection prevention personnel in any exercises where acceptable contamination criteria are determined. It is also important to note that not all areas and items will have the same contamination criteria. For example, certain areas of a hospital that house immunosuppressed patients may require sterile HCTs. These items must have zero contamination and thus must be handled differently than normal HCT. Incoming city water will most likely have different acceptable contamination levels than tunnel washer press tank water. Soil sort conveyer acceptable contamination levels will certainly be different from those on clean linen carts. The important practice is to define your acceptable contamination levels, measure to them, and then adjust/manage your process when actual levels fall outside of acceptable levels. Then repeat!
Putting it all together
Healthcare laundry linen monitoring is the surest way to ensure standard linen processes consistently provide hygienically clean and safe HCTs for sick patients. Best practices for implementing a laundry process monitoring program are built around:
Defining areas to test. We recommend hard surfaces, air, water, employee hands, and linen
Setting testing frequency. We recommend linen at least quarterly; other areas at least monthly.
Choosing a testing methodology.
Setting acceptable contamination levels across areas – We recommend defining these internally with your hospital infection prevention partners.
Managing to acceptable contamination levels – Make process, equipment, and people adjustments when acceptable levels are not met
Repeat the process
The end result: Hospital patients and their families will thank you for providing them with consistently clean linen and giving them one less thing to worry about as they heal!
In conclusion, the tenth and final installment of our "Unveiling the Unseen Threats" series underscores the indispensable role of process monitoring in ensuring the hygiene and safety of healthcare textiles. The urgency of rigorously monitoring every facet of healthcare textile processing cannot be overstated, as it plays a pivotal role in confirming a consistently clean outcome with each iteration. As we navigate the intricacies of healthcare textile cleaning, from the hospital environment to laundry facilities and transportation services, the importance of executing each step with precision becomes apparent. This vigilance is not merely a procedural requirement; it is a critical safeguard against potential linen contamination that could lead to severe consequences for patient health. The lack of agreed-upon standards of cleanliness in healthcare linen processes necessitates the implementation of a comprehensive process monitoring program, covering key areas such as surfaces, air, process water, employee hands, and the finished healthcare textiles. By defining areas to test, setting testing frequencies, choosing appropriate testing methodologies, and actively managing to acceptable contamination levels, healthcare facilities can consistently deliver hygienically clean and safe textiles for patients, providing them with peace of mind as they focus on their healing journey.